TESTICULAR TUMORS (35-39)
Testicular Tumors.
- Introduction and Incidence
- Classification
- EtioPathogenesis
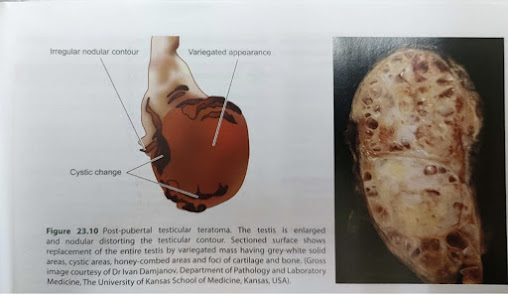
- Gross features and Microscopy
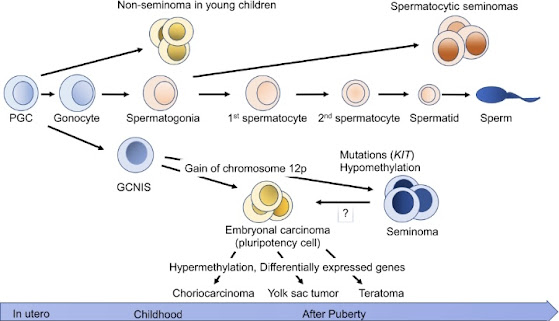
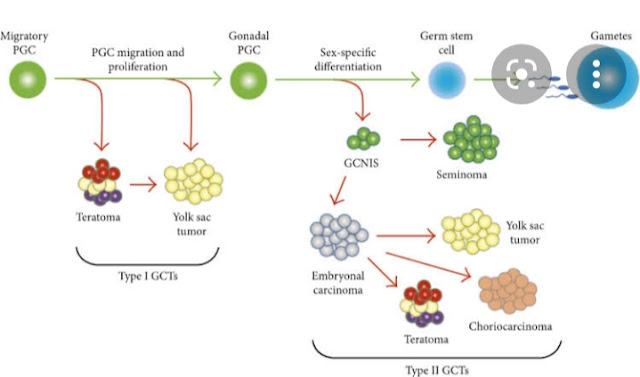
- Genetic Factors
- Histopathology
- Clinical Staging
- Clinical Features
- Risk Factors
- Differential Diagnosis.
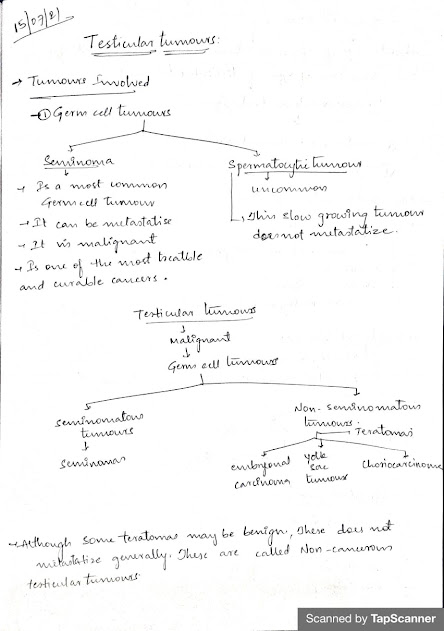
- The two main categories of testicular tumors are
- Germ cell tumors (95%) and
- Sex cord-stromal tumors.
- Germ cell tumors can start in several parts of the body:
- The testicles, which is the most common location
- The back of the abdomen near the spine, called the retroperitoneum.
- The central portion of the chest between the lungs, called the mediastinum.
- The lower spine.
- Very rarely, a small gland in the brain called the pineal gland.
- Age – most common solid tumor of men between 20-30 years
- Race – White : Black = 4:1 in U.S.
- Side – Right > Left
- Socio-economic status – high : low = 2:1 Geographical
- Highest in Scandinavia, Germany, Switzerland
- Intermediate – USA & UK Low – Africa and Asia
- 1. Seminoma: 35-40 yrs
- 2. Pure Terratoma: Pediatric age group
- 3. Embryonal CA: 25-30 yrs
- 4. Chorio CA: 25-35 yrs
- 5. Yolk sac Tumour: infancy & child hood
- 6. Mixed terrato CA: 25-30 yrs
- 7. Lymphoma: > 50 yrs
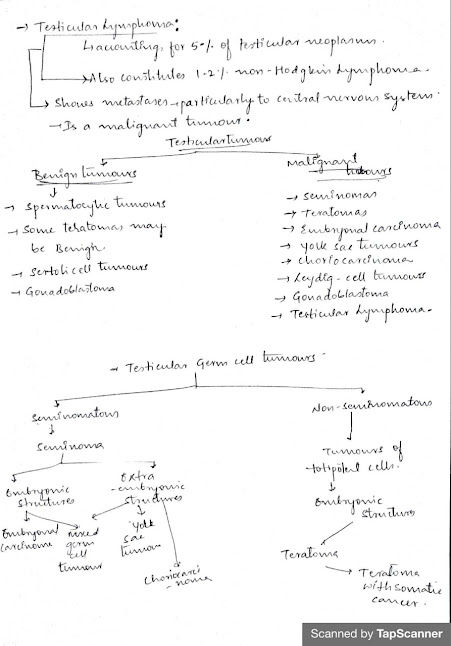
1. Noninvasive germ cell neoplasia
- Germ cell neoplasia in situ
- Specific forms of intratubular germ cell neoplasia
- Seminoma
- Seminoma with syncytiotrophoblast cells
- Embryonal carcinoma
- Yolk sac tumor, postpubertal type
- Trophoblastic tumors
- Choriocarcinoma
- Nonchoriocarcinomatous trophoblastic tumors
- Placental site trophoblastic tumor
- Epithelioid trophoblastic tumor
- Cystic trophoblastic tumor
- Teratoma, postpubertal type
- Teratoma with somatic type malignancy
- Mixed germ cell tumors
- Germ cell tumors of unknown type
- Regressed germ cell tumors
—Germ cell tumors unrelated to germ cell neoplasia in situ
2. Teratoma, prepubertal type
- Dermoid cyst
- Epidermoid cyst
- Well differentiated neuroendocrine tumor (monodermal teratoma)
—Sex cord stromal tumors
1. Pure tumors
- Leydig cell tumor
- Malignant Leydig cell tumor
- Sertoli cell tumor, NOS
- Malignant Sertoli cell tumor
- Large cell calcifying Sertoli cell tumor
- Intratubular large cell hyalinizing Sertoli cell neoplasia
- Granulosa cell tumor
- Adult granulosa cell tumor
- Juvenile granulosa cell tumor
- Tumors in the fibroma thecoma group
- Mixed sex cord stromal tumor
- Unclassified sex cord stromal tumor
—Tumor containing both germ cell and sex cord stromal elements
- Gonadoblastoma
—Miscellaneous tumors of the testis
- Ovarian epithelial type tumors
- Serous cystadenoma
- Serous tumor of borderline malignancy
- Serous cystadenocarcinoma
- Mucinous cystadenoma
- Mucinous borderline tumor
- Mucinous cystadenocarcinoma
- Endometrioid adenocarcinoma
- Clear cell adenocarcinoma
- Brenner tumor
- Juvenile xanthogranuloma
- Hemangioma
—Hematolymphoid tumors
- Diffuse large B cell lymphoma
- Follicular lymphoma, NOS
- Extranodal NK / T cell lymphoma, nasal type
- Plasmacytoma
- Myeloid sarcoma
- Rosai-Dorfman disease
—Tumors of collecting duct and rete testis
- Adenoma
- Adenocarcinoma
—Tumors of paratesticular structures
- Adenomatoid tumor
- Mesothelioma
- Well differentiated papillary mesothelioma
- Epididymal tumors
- Cystadenoma of the epididymis
- Papillary cystadenoma
- Adenocarcinoma of the epididymis
- Squamous cell carcinoma
- Melanotic neuroectodermal tumor
- Nephroblastoma
- Paraganglioma
—Mesenchymal tumors of the spermatic cord and testicular adnexa
- Lipoma
- Well differentiated liposarcoma
- Dedifferentiated liposarcoma
- Myxoid liposarcoma
- Pleomorphic liposarcoma
- Leiomyoma
- Leiomyosarcoma
- Rhabdomyoma
- Rhabdomyosarcoma
- Embryonal type
- Alveolar type
- Pleomorphic type
- Spindle cell / sclerosing type
- Cellular angiofibroma
- Mammary type myofibroblastoma
- Deep ("aggressive") angiomyxoma
 | ||||
| 1. Schiller-Duval bodies. 2. PAS-positive hyaline globules, many of which contain AFP. |
 |
| Histologically, the tumour is composed of sheets and cords of normal-looking Leydig cells. These cells contain abundant eosinophilic cytoplasm and Reinke’s crystals and a small central nucleus. |
- Stage I (A) – confined to testis with no spread through capsule or spermatic cord.
- Stage II (B) – Clinical or radiological evidence of spread beyond testis but with in regional L.N.
- Stage III (C) - Disseminated above diaphragm / visceral disease.
- Tumor (T): How large is the primary tumor? Where is it located?
- Node (N): Has the tumor spread to the lymph nodes in the back of the abdomen (retroperitoneum)?
- Metastasis (M): Has the cancer spread to other parts of the body? If so, where and how much?
- Serum tumor marker (S): Are the serum tumor markers AFP, beta-hCG, and LDH elevated? If so, how high are they?
- The usual presenting clinical symptoms of testicular tumours are: Gradual gonadal enlargement and a dragging sensation in the testis.
- Metastatic involvement may produce secondary symptoms such as pain, lymphadenopathy, haemoptysis and urinary obstruction.
- Lymphatic spread occurs to retroperitoneal para-aortic lymph nodes, mediastinal lymph nodes and supraclavicular lymph nodes.
- Haematogenous spread primarily occurs to the lungs, liver, brain and bones.
- Two tumour markers widely used in the diagnosis, staging and monitoring the follow-up of patients with testicular tumours are: Human chorionic gonadotropin (hCG) and Alpha-foetoprotein (AFP).
- In addition, carcinoembryonic antigen (CEA), human placental lactogen (HPL), placental alkaline phosphatase, testosterone, oestrogen and luteinising hormone may also be elevated.
- hCG is synthesised by placental syncytio-trophoblast such as in various non-seminomatous germ cell tumours of the testis (e.g. in choriocarcinoma, yolk sac tumour and embryonal carcinoma). However, ectopic hCG production may occur in a variety of non-testicular non-germ cell tumours as well.
- AFP is normally synthesised by the foetal liver cells, yolk sac and foetal gut. Its levels are elevated in testicular tumours associated with yolk sac components. However, elevated serum AFP levels are also found in liver cell carcinoma.
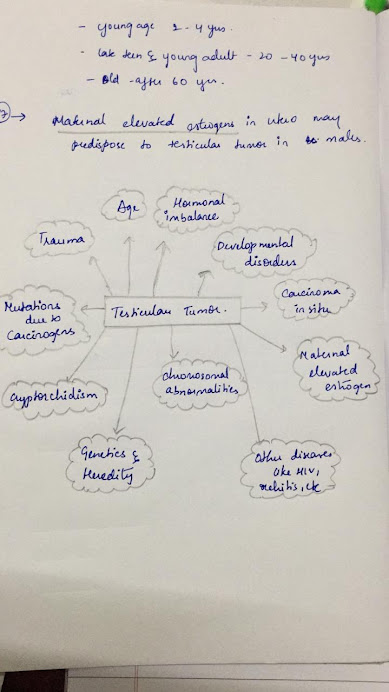
The following factors can raise a person’s risk of developing testicular cancer. However, it is important to note that the cause of testicular cancer is not known.
Age. More than half of the people who are diagnosed with testicular cancer are between age 20 and 45. However, people of any age can develop this disease, including those in their teens and in their 60s.
Cryptorchidism. Cryptorchidism is an undescended testicle, meaning that 1 or both testicles do not move down into the scrotum before birth. This condition increases the risk of developing testicular cancer.
Family history. A person who has a close relative, particularly a sibling, who has had testicular cancer has an increased risk of developing testicular cancer.
Personal history. People who have had cancer in 1 testicle have an increased risk of developing cancer in the other testicle.
Race. Although people of any race can develop testicular cancer, white people are more likely than those of other races to be diagnosed with testicular cancer.
Human immunodeficiency virus (HIV). Slightly higher risk of developing seminoma.
DIFFERENTIAL DIAGNOSIS
- Epidedymo-orchitis
- Testicular haematoma
- Spermatocele
- Hydrocele
- Testicular Torsion
- Haematological – Hb%, Bl. urea/S. creatinine, LFT
- Tumour markers – AFP, HCG, LDH
- Scrotal Ultrasound – Usually homogenous,
- hypoechoic, intra testicular mass
- X-ray chest
- CT / MRI – abdomen
Harsh Mohan tb of pathology
Robbins tb of pathology
Internet.
























Comments
Post a Comment